Long-term ALPHA Phase III trial data showed danicopan as add-on to ULTOMIRIS® or SOLIRIS® sustained clinical improvements in subset of patients with PNH experiencing clinically significant extravascular hemolysis

Data demonstrated effective control of intravascular and extravascular hemolysis through 48 weeks
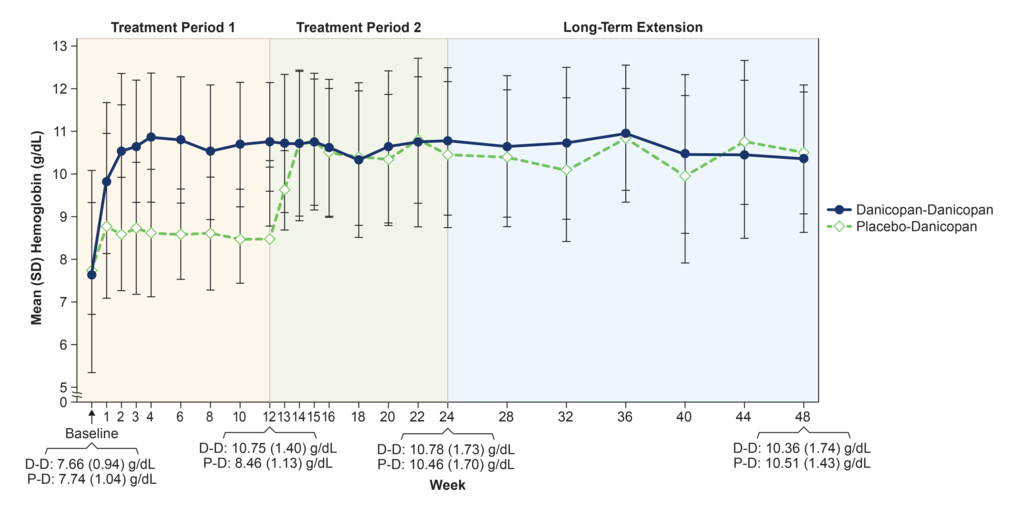
Results showed increase in mean hemoglobin levels were maintained through 48 weeks
WILMINGTON, Del.–(BUSINESS WIRE)–Positive results from the 24-week and long-term extension (LTE) period of the pivotal ALPHA Phase III trial showed danicopan as add-on to standard of care C5 inhibitor therapy ULTOMIRIS® (ravulizumab-cwvz) or SOLIRIS® (eculizumab) continued to demonstrate clinical benefit for patients with paroxysmal nocturnal hemoglobinuria (PNH) who experience clinically significant extravascular hemolysis (EVH).1
Results from the trial were presented today at the 65th American Society of Hematology (ASH) Annual Meeting and Exposition in San Diego, California. Danicopan is an investigational, first-in-class, oral, Factor D inhibitor.
Data showed that improvements in mean hemoglobin levels and absolute reticulocyte count (ARC) levels, which were demonstrated at 12 weeks, were maintained through 48 weeks.1
PNH is a rare and severe blood disorder characterized by the destruction of red blood cells within blood vessels, known as intravascular hemolysis (IVH), and white blood cell and platelet activation that can cause thrombosis (blood clots) and result in organ damage and potentially premature death.2-4 Immediate, complete and sustained terminal complement inhibition by blocking the C5 protein with ULTOMIRIS or SOLIRIS helps reduce symptoms and complications, resulting in improved survival for patients with PNH.4-7 Approximately 10-20% of people living with PNH who are treated with a C5 inhibitor experience clinically significant EVH, which can result in continued symptoms of anemia and require blood transfusions.2,8-12
Austin Kulasekararaj, MD, Consultant Hematologist at King’s College Hospital, London and investigator in the ALPHA trial, said: “These new data further demonstrate the potential of danicopan as add-on to ULTOMIRIS or SOLIRIS to address the needs of the small subset of patients with PNH who experience clinically significant EVH. Expanding on positive 12-week results, the findings demonstrate sustained improvements in hemoglobin levels for up to 48 weeks, while also maintaining disease control, as measured by lactate dehydrogenase levels.”
Gianluca Pirozzi, Senior Vice President, Head of Development, Regulatory and Safety, Alexion, said: “Unlike IVH, EVH is not life-threatening, but its manifestations can be burdensome for people living with this condition, which is why we continue to explore the potential of the complement system to advance patient care. The pivotal ALPHA results suggest that dual complement pathway inhibition at Factor D and C5 may be an optimal treatment approach for the 10-20% of patients with PNH who experience clinically significant EVH. Importantly, C5 inhibition maintains effective IVH control, which is critical for patients, and the addition of Factor D inhibition addresses signs and symptoms of EVH.”
The pivotal ALPHA Phase III trial is designed as a superiority study to evaluate the efficacy and safety of danicopan as an add-on to C5 inhibitor therapy ULTOMIRIS or SOLIRIS in patients with PNH who experience clinically significant EVH. A total of 86 patients were randomized. The prespecified interim analysis (primary analysis) occurred after 63 participants either completed or discontinued from the primary treatment period of 12 weeks. Following the 12-week randomized control period, patients were eligible to enroll in an open-label treatment period for an additional 12 weeks. During the open-label period, participants receiving placebo plus ULTOMIRIS or SOLIRIS switched to danicopan plus ULTOMIRIS or SOLIRIS (placebo-danicopan), and participants receiving add-on therapy with danicopan continued treatment with danicopan add-on therapy (danicopan-danicopan). The open-label treatment period was followed by the option to join a two-year LTE period during which all participants received danicopan add-on therapy. At the time of data cut-off on September 20, 2022, 60 of the 63 patients who were included in the primary analysis had reached 24 weeks and entered the LTE.1
Data showed that the significant improvements in hemoglobin levels observed at 12 weeks [LSM (SEM) change 2.94 (0.21) g/dL] continued at 24 weeks [LSM (SEM) change 3.17 (0.30) g/dL] among patients treated with danicopan plus ULTOMIRIS or SOLIRIS and were sustained through 48 weeks.1
Secondary endpoints measured at 24 weeks include change from baseline in hemoglobin, ARC, and lactate dehydrogenase (LDH) levels; the percentage of patients with hemoglobin increase of ≥2 g/dL in the absence of transfusion; and the percentage of patients with transfusion avoidance.1
All key secondary endpoints met superiority in favor of danicopan plus ULTOMIRIS or SOLIRIS compared to placebo plus ULTOMIRIS or SOLIRIS at 12 weeks, and data showed benefits were maintained at 24 weeks in the danicopan-danicopan arm.1
Further, all key secondary endpoints showed meaningful improvement at 24 weeks in patients who switched from placebo to add-on treatment with danicopan at 12 weeks, including ARC levels and percentage of patients with transfusion avoidance, two indicators of potential EVH.1
Additionally, mean (SD) LDH levels were maintained from baseline through 48 weeks in both treatment arms, demonstrating effective control of terminal complement activity and IVH with ULTOMIRIS or SOLIRIS.1
Summary of efficacy resultsi
Hemoglobin and ARC levels improved with danicopan at 12 weeks and were maintained through 48 weeks (please see hemoglobin and ARC level graphs included in the image carousel).
| Endpoints | Statistic | Danicopan-Danicopan | Placebo-Danicopan | ||
| Change at | Change at | Change at | Change at | ||
| Change from baseline in hemoglobin (g/dL) | LSM (SEM) | 2.94 (0.21) | 3.17 (0.30) | 0.50 (0.31) | 2.26 (0.34) |
| Change from baseline in ARC levels (×109/L) | LSM (SEM) | –83.8 (8.93) | –80.2 (8.75) | 3.5 (12.68) | –65.2 (12.74) |
| Change from baseline in LDH levels (U/L) | LSM (SEM) | –23.49 (8.29) | –17.79 (13.73) | –2.92 (11.91) | –6.03 (18.77) |
| Endpoints | Statistic | Danicopan-Danicopan | Placebo-Danicopan | ||
| Percent at | Percent at | Percent at | Percent at | ||
| Proportion of participants with transfusion avoidance (%) | Percent (%) | 83 | 78 | 38 | 90 |
i. LDH, lactate dehydrogenase; ARC, absolute reticulocyte count; LSM, least squares mean; SEM, standard error of the mean; ULN, upper limit of normal.
Results from the ALPHA Phase III trial and LTE showed danicopan is generally well tolerated, and no new safety concerns were identified. The safety analysis was performed using data from all participants who took at least one dose of danicopan (n=80). The most common treatment-emergent adverse events (TEAEs) (≥10%) were COVID-19 (21.3%), diarrhea (15%), headache (15%), pyrexia (13.8%), nausea (12.5%) and fatigue (10%).1
Additionally, an analysis of patient-reported outcomes from the ALPHA Phase III trial at 24 weeks was also presented at ASH, suggesting danicopan plus ULTOMIRIS or SOLIRIS has the potential to improve quality of life compared to C5 inhibitor therapy alone for the 10-20% of patients with PNH who experience clinically significant EVH.13 Findings showed clinically relevant patient-reported outcomes were observed in patients treated with danicopan as add-on to ULTOMIRIS or SOLIRIS during the first 12 weeks of treatment, compared to placebo plus C5 inhibition. Additionally, data showed improvements in Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) and European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 Scale (EORTC-QLQ-C30) scores were maintained during the open-label period to 24 weeks in the danicopan-danicopan arm and improved at 24 weeks in the placebo-danicopan arm.13
ALPHA Phase III trial results from the primary prespecified interim analysis at 12 weeks were presented at the European Hematology Association (EHA) 2023 Hybrid Congress and published in The Lancet Haematology.
Regulatory submissions for danicopan are currently under review with multiple global health authorities.
INDICATION(S) & IMPORTANT SAFETY INFORMATION for ULTOMIRIS® (ravulizumab-cwvz)
What is ULTOMIRIS?
ULTOMIRIS is a prescription medicine used to treat:
- adults and children 1 month of age and older with a disease called Paroxysmal Nocturnal Hemoglobinuria (PNH).
- adults and children 1 month of age and older with a disease called atypical Hemolytic Uremic Syndrome (aHUS). ULTOMIRIS is not used in treating people with Shiga toxin E. coli related hemolytic uremic syndrome (STEC-HUS).
- adults with a disease called generalized Myasthenia Gravis (gMG) who are anti-acetylcholine receptor (AChR) antibody positive.
- adults with PNH or aHUS when administered subcutaneously (under your skin).
It is not known if ULTOMIRIS is safe and effective in children younger than 1 month of age.
It is not known if ULTOMIRIS is safe and effective for the treatment of gMG in children.
Subcutaneous administration of ULTOMIRIS has not been evaluated and is not approved for use in children.
IMPORTANT SAFETY INFORMATION
What is the most important information I should know about ULTOMIRIS?
ULTOMIRIS is a medicine that affects your immune system and can lower the ability of your immune system to fight infections.
- ULTOMIRIS increases your chance of getting serious and life-threatening meningococcal infections that may quickly become life-threatening and cause death if not recognized and treated early.
- You must receive meningococcal vaccines at least 2 weeks before your first dose of ULTOMIRIS if you are not vaccinated.
- If your healthcare provider decided that urgent treatment with ULTOMIRIS is needed, you should receive meningococcal vaccination as soon as possible.
- If you have not been vaccinated and ULTOMIRIS therapy must be initiated immediately, you should also receive 2 weeks of antibiotics with your vaccinations.
- If you had a meningococcal vaccine in the past, you might need additional vaccination. Your healthcare provider will decide if you need additional vaccination.
- Meningococcal vaccines reduce but do not prevent all meningococcal infections. Call your healthcare provider or get emergency medical care right away if you get any of these signs and symptoms of a meningococcal infection: headache with nausea or vomiting, headache and fever, headache with a stiff neck or stiff back, fever, fever and a rash, confusion, muscle aches with flu-like symptoms and eyes sensitive to light.
Your healthcare provider will give you a Patient Safety Card about the risk of meningococcal infection. Carry it with you at all times during treatment and for 8 months after your last ULTOMIRIS dose. It is important to show this card to any healthcare provider or nurse to help them diagnose and treat you quickly.
ULTOMIRIS is only available through a program called the ULTOMIRIS REMS. Before you can receive ULTOMIRIS, your healthcare provider must: enroll in the ULTOMIRIS REMS program; counsel you about the risk of meningococcal infection; give you information and a Patient Safety Card about the symptoms and your risk of meningococcal infection (as discussed above); and make sure that you are vaccinated with a meningococcal vaccine, and if needed, get revaccinated with the meningococcal vaccine. Ask your healthcare provider if you are not sure if you need to be revaccinated.
ULTOMIRIS may also increase the risk of other types of serious infections. Make sure your child receives vaccinations against Streptococcus pneumoniae and Haemophilus influenzae type b (Hib) if treated with ULTOMIRIS. Call your healthcare provider right away if you have any new signs or symptoms of infection.
Who should not receive ULTOMIRIS?
Do not receive ULTOMIRIS if you have a meningococcal infection or have not been vaccinated against meningococcal infection unless your healthcare provider decides that urgent treatment with ULTOMIRIS is needed.
Before you receive ULTOMIRIS, tell your healthcare provider about all of your medical conditions, including if you: have an infection or fever, are pregnant or plan to become pregnant, and are breastfeeding or plan to breastfeed. It is not known if ULTOMIRIS will harm your unborn baby or if it passes into your breast milk. You should not breastfeed during treatment and for 8 months after your final dose of ULTOMIRIS.
Tell your healthcare provider about all the vaccines you receive and medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements which could affect your treatment.
If you have PNH and you stop receiving ULTOMIRIS, your healthcare provider will need to monitor you closely for at least 16 weeks after you stop ULTOMIRIS. Stopping ULTOMIRIS may cause breakdown of your red blood cells due to PNH. Symptoms or problems that can happen due to red blood cell breakdown include: drop in your red blood cell count, tiredness, blood in your urine, stomach-area (abdomen) pain, shortness of breath, blood clots, trouble swallowing, and erectile dysfunction (ED) in males.
If you have aHUS, your healthcare provider will need to monitor you closely for at least 12 months after stopping treatment for signs of worsening aHUS or problems related to a type of abnormal clotting and breakdown of your red blood cells called thrombotic microangiopathy (TMA). Symptoms or problems that can happen with TMA may include: confusion or loss of consciousness, seizures, chest pain (angina), difficulty breathing and blood clots or stroke.
ULTOMIRIS can cause serious side effects including allergic reactions to acrylic adhesive. Allergic reactions to the acrylic adhesive may happen with your subcutaneous ULTOMIRIS treatment. If you have an allergic reaction during the delivery of subcutaneous ULTOMIRIS, remove the on-body injector and get medical help right away. Your healthcare provider may treat you with medicines to help prevent or treat allergic reaction symptoms as needed.
What are the possible side effects of ULTOMIRIS?
ULTOMIRIS can cause serious side effects including infusion-related reactions. Symptoms of an infusion-related reaction with ULTOMIRIS may include lower back pain, tiredness, feeling faint, discomfort in your arms or legs, bad taste, or drowsiness. Stop treatment of ULTOMIRIS and tell your healthcare provider or nurse right away if you develop these symptoms, or any other symptoms during your ULTOMIRIS infusion that may mean you are having a serious infusion reaction, including: chest pain, trouble breathing or shortness of breath, swelling of your face, tongue, or throat, and feel faint or pass out.
The most common side effects of ULTOMIRIS in people treated for PNH are upper respiratory tract infection and headache.
The most common side effects of ULTOMIRIS in people treated for aHUS are upper respiratory tract infection, diarrhea, nausea, vomiting, headache, high blood pressure and fever.
The most common side effects of ULTOMIRIS in people with gMG are diarrhea and upper respiratory tract infections.
The most common side effects of subcutaneous administration of ULTOMIRIS in adults treated for PNH and aHUS are local injection site reactions.
Tell your healthcare provider about any side effect that bothers you or that does not go away. These are not all the possible side effects of ULTOMIRIS. For more information, ask your healthcare provider or pharmacist. Call your healthcare provider right away if you miss an ULTOMIRIS infusion or for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Read the Instructions for Use that comes with subcutaneous ULTOMIRIS for instructions about the right way to prepare and give your subcutaneous ULTOMIRIS injections through an on-body injector.
Please see the accompanying full Prescribing Information and Medication Guide for ULTOMIRIS, including Boxed WARNING regarding serious and life-threatening meningococcal infections/sepsis. Please see the accompanying Instructions for Use for the ULTOMIRIS On Body Delivery System.
INDICATIONS & IMPORTANT SAFETY INFORMATION FOR SOLIRIS® (eculizumab) [injection for intravenous use 300mg/30mL vial]
What is SOLIRIS?
SOLIRIS is a prescription medicine used to treat:
- patients with a disease called Paroxysmal Nocturnal Hemoglobinuria (PNH).
- adults and children with a disease called atypical Hemolytic Uremic Syndrome (aHUS). SOLIRIS is not for use in treating people with Shiga toxin E. coli related hemolytic uremic syndrome (STEC-HUS).
- adults with a disease called generalized myasthenia gravis (gMG) who are anti-acetylcholine receptor (AChR) antibody positive.
- adults with a disease called neuromyelitis optica spectrum disorder (NMOSD) who are anti-aquaporin-4 (AQP4) antibody positive.
It is not known if SOLIRIS is safe and effective in children with PNH, gMG, or NMOSD.
IMPORTANT SAFETY INFORMATION
What is the most important information I should know about SOLIRIS?
SOLIRIS is a medicine that affects your immune system and can lower the ability of your immune system to fight infections.
- SOLIRIS increases your chance of getting serious and life-threatening meningococcal infections that may quickly become life-threatening and cause death if not recognized and treated early.
- You must receive meningococcal vaccines at least 2 weeks before your first dose of SOLIRIS if you are not vaccinated.
- If your doctor decided that urgent treatment with SOLIRIS is needed, you should receive meningococcal vaccination as soon as possible.
- If you have not been vaccinated and SOLIRIS therapy must be initiated immediately, you should also receive 2 weeks of antibiotics with your vaccinations.
- If you had a meningococcal vaccine in the past, you might need additional vaccination. Your doctor will decide if you need additional vaccination.
- Meningococcal vaccines reduce but do not prevent all meningococcal infections. Call your doctor or get emergency medical care right away if you get any of these signs and symptoms of a meningococcal infection: headache with nausea or vomiting, headache and fever, headache with a stiff neck or stiff back, fever, fever and a rash, confusion, muscle aches with flu-like symptoms, and eyes sensitive to light.
Your doctor will give you a Patient Safety Card about the risk of meningococcal infection. Carry it with you at all times during treatment and for 3 months after your last SOLIRIS dose. It is important to show this card to any doctor or nurse to help them diagnose and treat you quickly.
SOLIRIS is only available through a program called the SOLIRIS REMS. Before you can receive SOLIRIS, your doctor must enroll in the SOLIRIS REMS program; counsel you about the risk of meningococcal infection; give you information and a Patient Safety Card about the symptoms and your risk of meningococcal infection (as discussed above); and make sure that you are vaccinated with the meningococcal vaccine and, if needed, get revaccinated with the meningococcal vaccine. Ask your doctor if you are not sure if you need to be revaccinated.
SOLIRIS may also increase the risk of other types of serious infections. Make sure your child receives vaccinations against Streptococcus pneumoniae and Haemophilus influenzae type b (Hib) if treated with SOLIRIS. Certain people may be at risk of serious infections with gonorrhea. Certain fungal infections (Aspergillus) may occur if you take SOLIRIS and have a weak immune system or a low white blood cell count.
Who should not receive SOLIRIS?
Do not receive SOLIRIS if you have a meningococcal infection or have not been vaccinated against meningitis infection unless your doctor decides that urgent treatment with SOLIRIS is needed.
Before you receive SOLIRIS, tell your doctor about all of your medical conditions, including if you: have an infection or fever, are pregnant or plan to become pregnant, and are breastfeeding or plan to breastfeed. It is not known if SOLIRIS will harm your unborn baby or if it passes into your breast milk.
Tell your doctor about all the vaccines you receive and medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements which could affect your treatment. It is important that you have all recommended vaccinations before you start SOLIRIS, receive 2 weeks of antibiotics if you immediately start SOLIRIS, and stay up-to-date with all recommended vaccinations during treatment with SOLIRIS.
If you have PNH, your doctor will need to monitor you closely for at least 8 weeks after stopping SOLIRIS. Stopping treatment with SOLIRIS may cause breakdown of your red blood cells due to PNH. Symptoms or problems that can happen due to red blood cell breakdown include: drop in the number of your red blood cell count, drop in your platelet count, confusion, kidney problems, blood clots, difficulty breathing, and chest pain.
If you have aHUS, your doctor will need to monitor you closely during and for at least 12 weeks after stopping treatment for signs of worsening aHUS symptoms or problems related to abnormal clotting (thrombotic microangiopathy). Symptoms or problems that can happen with abnormal clotting may include: stroke, confusion, seizure, chest pain (angina), difficulty breathing, kidney problems, swelling in arms or legs, and a drop in your platelet count.
What are the possible side effects of SOLIRIS?
SOLIRIS can cause serious side effects including serious infusion-related reactions. Tell your doctor or nurse right away if you get any of these symptoms during your SOLIRIS infusion: chest pain; trouble breathing or shortness of breath; swelling of your face, tongue, or throat; and feel faint or pass out. If you have an infusion-related reaction to SOLIRIS, your doctor may need to infuse SOLIRIS more slowly, or stop SOLIRIS.
The most common side effects in people with PNH treated with SOLIRIS include: headache, pain or swelling of your nose or throat (nasopharyngitis), back pain, and nausea.
The most common side effects in people with aHUS treated with SOLIRIS include: headache, diarrhea, high blood pressure (hypertension), common cold (upper respiratory infection), stomach-area (abdominal) pain, vomiting, pain or swelling of your nose or throat (nasopharyngitis), low red blood cell count (anemia), cough, swelling of legs or feet (peripheral edema), nausea, urinary tract infections, and fever.
The most common side effects in people with gMG treated with SOLIRIS include: muscle and joint (musculoskeletal) pain.
The most common side effects in people with NMOSD treated with SOLIRIS include: common cold (upper respiratory infection); pain or swelling of your nose or throat (nasopharyngitis); diarrhea; back pain; dizziness; flu-like symptoms (influenza), including fever, headache, tiredness, cough, sore throat, and body aches; joint pain (arthralgia); throat irritation (pharyngitis); and bruising (contusion).
Tell your doctor about any side effect that bothers you or that does not go away. These are not all the possible side effects of SOLIRIS. For more information, ask your doctor or pharmacist. Call your doctor for medical advice about side effects. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit MedWatch, or call 1-800-FDA-1088.
Please see the full Prescribing Information and Medication Guide for SOLIRIS, including Boxed WARNING regarding serious and life-threatening meningococcal infections.
Notes
PNH
PNH is a rare, chronic, progressive and potentially life-threatening blood disorder.
Contacts
Media Inquiries
Brendan McEvoy
+1 302 885 2677
US Media Mailbox: [email protected]