AVM Biotechnology Selected by the National Cancer Institute to Present at Bio Investor Forum 2023

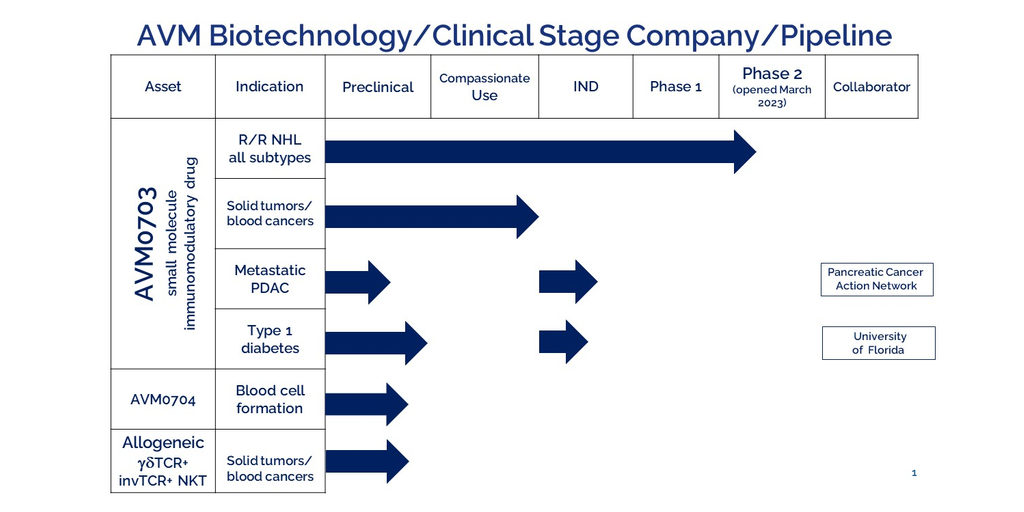
SEATTLE–(BUSINESS WIRE)–#AMSM—AVM Biotechnology, a clinical stage company enrolling Phase 2 adaptive design/expansion cohorts for immunomodulatory AVM0703 in relapsed/refractory Non-Hodgkin Lymphoma/Leukemia patients, will present at the Bio Investor Forum, held in San Francisco, CA October 17-18, 2023. AVM Biotechnology’s pipeline includes the small molecule AVM0703 with applications to solid tumors as well as blood cancers, small molecule AVM0704 to induce blood cell formation to reduce need for transfusions and granulocyte-colony stimulating factor treatments, and an allogeneic ‘off-the shelf’ bispecific Natural Killer T-like cell that expresses both the gamma delta TCR and the invariant TCR.
In June 2023, AVM Biotechnology was selected as a 2023-2024 National Cancer Institute (NCI) Showcase Company for their Small Business Innovation Research (SBIR) Investor Initiatives. AVM Biotechnology has previously been awarded five (5) highly competitive National Institute of Health SBIR grants; three (3) of these from the National Cancer Institute.
About AVM Biotechnology Inc:
AVM Biotechnology is a clinical stage company headquartered in Seattle, WA developing immunomodulatory therapies for hematological cancer, infectious and autoimmune diseases. AVM’s lead drug, immunomodulatory AVM0703, has demonstrated relatively broad anti-cancer activity against a variety of solid tumors and blood cancers. Over 57 patients have been treated to date. AVM0703’s relatively broad anti-cancer activity is hypothesized to be due to mobilization of a highly active gamma/delta T-cell receptor expressing immune cell, which is programmed to recognize special stress signals produced by most cancer cells but not normal cells. Requests for additional information about immunomodulatory AVM0703 and its relatively broad activity against solid tumors and blood cancers can be made by contacting [email protected] and for information about our enrolling clinical trial for relapsed/refractory Non-Hodgkin’s Lymphoma/Leukemia patients please contact [email protected] or [email protected]. AVM’s pipeline also includes a small molecule that triggers blood cell formation to replace transfusions, and an allogeneic γδTCR+invTCR+ NKT-like cell therapy.
About AVM0703:
AVM0703 is small molecule immunomodulatory drug enrolling Phase 2 trials in US in relapsed/refractory Non-Hodgkin’s Lymphoma (NHL) which began enrollment H1 2023 (partially funded by NCI Ph II FastTrak grant 1R44CA272096). AVM0703 mobilizes a novel endogenous bispecific gamma delta TCR+ invariant TCR+ Natural Killer T-like cell with profound antitumor activity. AVM0703 has shown an absence of safety concerns with side-effects limited to grades 1-3. Clinical responses in the enrolling NHL trial and in FDA-approved expanded access/compassionate use include multiple NHL sub-types and diverse solid tumor types. Responses to AVM0703 are quite rapid, reported from 30 minutes to 14 days after infusion. Preclinical data also demonstrates a significant response against autoreactive lymphocytes in the NOD Type 1 diabetes model (Funded by NIDDK SBIR Ph I grant 1R43DK121634 and NIDDK SBIR Ph II grant 2R44DK121634). Gamma delta TCR+ lymphocytes recognize phosphoantigens expressed by stressed, cancer and infected cells and autoreactive lymphocytes, but not normal cells. Adoptive transfer of AVM0703 induced gamma delta TCR+ immune cells has potent activity against preclinical melanoma (funded by NCI SBIR Ph I grant 1R43CA246896). Additionally, AVM0703 has been shown to have potent neo-adjuvant activity before chemotherapy against immune-resistant, aggressive mouse A20 lymphoma (Funded by NCI SBIR Ph I 1R43_CA271951). Based on its ability to penetrate collagen-encased desmoplastic tumors, AVM0703 has promise as a neoadjuvant prior to chemoimmunotherapy to improve outcomes for metastatic advanced pancreatic cancer patients.
Forward Looking Statements: This contains certain statements that constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. These statements do not relate strictly to historical or current facts and they may be accompanied by words such as “could,” “would,” “may,” “potentially,” “suggest,” “believes,” “expects,” “should,” and similar words or expressions. These forward-looking statements reflect our current views as of the date this is published, and are subject to risks, uncertainties, assumptions, changes in circumstances, and other factors; drug development and commercialization are highly risky and early clinical results in animals or humans may not reflect the full results from later stage or larger scale clinical trials. These forward-looking statements are subject to risks and uncertainties that could cause our actual results, performance, and expectations to differ materially from those expressed or implied by these statements, including statements about: future and ongoing drug development and timing; the applications of drugs to specific diseases; the potential for ongoing preclinical or clinical trial results; FDA or other regulatory findings and approvals; potential market opportunities; and the occurrence of future events or circumstances. There are risks and uncertainties involving and not limited to our ability to progress in our research and development efforts, complete clinical testing, achieve our expected results, commercialize our products, avoid infringement of patents, trademarks and other proprietary rights of third parties, protect products from competition, navigate the political environment, maintain sufficient capital and funding, avoid problems with our manufacturing processes, maintain our operations, and obtain regulatory approval to sell and market the drugs in the United States and elsewhere. The reader should not place any undue reliance on such forward-looking statements. We have no obligation to release publicly the results of any revisions to any of our forward-looking statements to reflect events or circumstances after the date these statements are made or to reflect the occurrence of unanticipated events, except as may be required by law.
Contacts
AVM Biotechnology
Theresa Deisher
[email protected]
[email protected]
Compassionate Use
Mia Lor
[email protected]
Partnering/Investing
Todd Bertsch
[email protected]