22 states doing comprehensive testing as per WHO guidance, of more than 140 per day per million population : Health Ministry
Health Ministry has started a consultative exercise to gather information from treating doctors, on complications being faced by patients who have recovered from COVID-19
Number of people in India who have recovered from COVID-19 is around 1.8 times the number of active cases. In many states, the number of daily discharges from hospitals is more than that of daily admissions. This apart, 86% of total active COVID-19 cases are confined to just 10 states, while 50% of active cases are in two states, which shows that spread of COVID-19 is not happening uniformly across the nation. This has been stated by Shri Rajesh Bhushan, Officer-on-Special Duty (OSD), Ministry of Health and Family Welfare, in course of a press briefing on actions taken, preparedness and updates on COVID-19 at New Media Centre, in New Delhi today. The presentation given by the Ministry can be accessed here.
Shri Bhushan also stated:
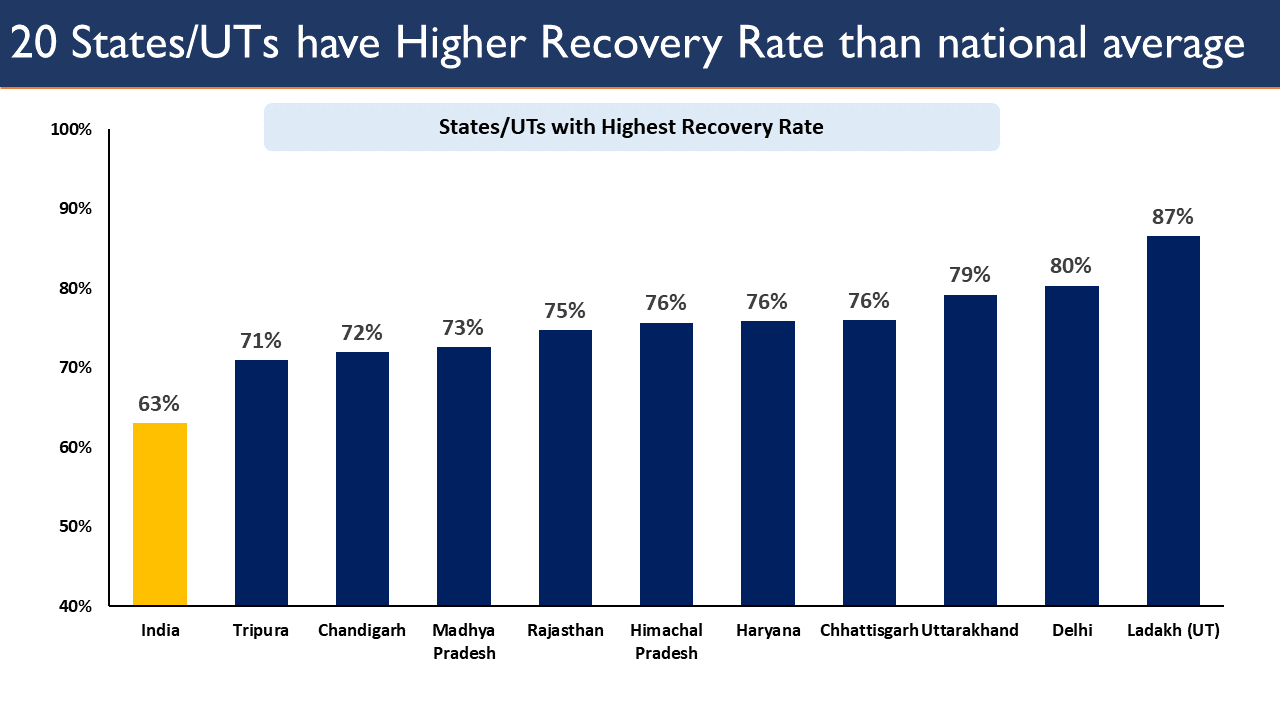
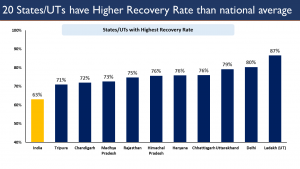
- National COVID-19 recovery rate of India has been continuously increasing.
- 20 states / UTs have a higher COVID-19 recovery rate than the national rate.
- Till the end of May 2020, number of active COVID-19 cases was higher than recovered cases. But after that, the number of recovered cases has become more than active cases and the gap between the two is increasing.
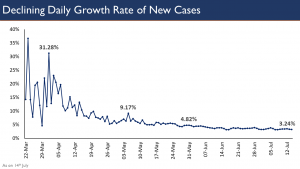
Shri Bhushan further informed that number of COVID-19 cases per million in India is amongst the lowest in the world. The figure in some countries is around 7 – 14 times higher than that of India. Deaths per million population in India too is amongst the lowest in the world, while in some countries it is 35 times higher. Stating these, the OSD said, “A focus only on absolute numbers tends to cloud our policy focus”. Despite being the second most populous country of the world, the daily growth rate of new COVID-19 cases in India has been coming down continuously, he added.
Speaking on the matter of COVID-19 testing, Shri Bhushan said, World Health Organisation has come out with a document underlining need for comprehensive surveillance and testing of suspect COVID-19 cases. In that document, it is mentioned that testing 140 people per day per 10 lakhs would be indicative of ‘comprehensive’. In this context, Shri Bhushan stated, 22 states are doing more than 140 COVID-19 tests per day per million. Centre is asking states and UTs to increase testing so that we proceed towards the guidelines given by WHO, he added. There were 101 COVID19 RT-PCR testing labs in mid-March, whereas today there are 1,206 RT-PCR testing labs and 280 Rapid Antigen Test centres. This has significantly increased our testing capacity.
 The OSD, Health Ministry further said, focus on containment, testing and clinical management needs to be continued. Urging that there is a need for community ownership of the fight against COVID-19, he said, Health Ministry has laid out the steps to be taken to manage COVID-19 going forward.
The OSD, Health Ministry further said, focus on containment, testing and clinical management needs to be continued. Urging that there is a need for community ownership of the fight against COVID-19, he said, Health Ministry has laid out the steps to be taken to manage COVID-19 going forward.
 Prof. (Dr.) Balram Bhargava, DG, ICMR said, 60% of the vaccines supplied in the world are of Indian origin and added, India is perceived and is an important player in the vaccine supply for the world. He further stated, any COVID-19 vaccine produced or developed in any part of the world will ultimately have to be scaled up either by India or by China. Every country which is developing vaccine is in communication with India since they are aware India is a major producer, said the DG of ICMR.
Prof. (Dr.) Balram Bhargava, DG, ICMR said, 60% of the vaccines supplied in the world are of Indian origin and added, India is perceived and is an important player in the vaccine supply for the world. He further stated, any COVID-19 vaccine produced or developed in any part of the world will ultimately have to be scaled up either by India or by China. Every country which is developing vaccine is in communication with India since they are aware India is a major producer, said the DG of ICMR.
Prof. (Dr.) Bhargava also said, two indigenous Indian vaccine candidates have undergone successful toxicity studies in rats, mice and rabbits and the toxicity data has been shared with DCGI. They have got clearance to start human trials early this month. Approximately 1,000 human volunteers are participating in clinical trials for each of the two indigenous vaccine candidates and pre-clinical experiments for these as well as other vaccines are also being done at NIV, Pune, he further stated.
In the context of fast-tracking COVID-19 vaccine development, the DG, ICMR said, it is a moral duty. Russia and China have fast-tracked the process, USA and UK too are trying to fast-track the development programme. India too is trying to fast-track its two indigenous vaccine candidates, he added. Every country has to work together to develop these vaccines for everyone so that the chain of transmission of the pandemic can be arrested. Efforts are being taken to ensure that not a single day is wasted for regulatory or approval purpose, without compromising on the science, quality and ethics part of it, he further stated.
In reply to a media query, Shri Bhushan stated that Health Ministry has started a consultative exercise at the level of DG, Health Services, to gather information from treating doctors, on complications being faced by patients who have recovered from COVID19, based on which, some guidelines may be issued in the future.
The national COVID19 fatality rate is 2.6% and it is coming down. It is significantly lower than global fatality rate, further stated the OSD, Health Ministry in reply to a question. Since the last two weeks, AIIMS doctors has been engaging two days every week with ICU doctors of treating hospitals to resolve problems faced in saving critically ill patients, he further informed.
In response to another media query, Shri Bhushan answered that people in home isolation are monitored by paramedical workers and volunteers who are given the responsibility to check these people on a daily basis and then they submit a report based on set parameters based on which a decision is taken about continuation of home isolation or shifting patients to a COVID-Care facility. Secondly, dedicated home-health care agencies may also make telephone calls and check upon the patients in home-isolation on the basis of a similar check-list.
Emergency approval by drugs regulator can be given on the use of any drug based on restricted evidence by a drug-maker to a regulator. It is distinct from market authorisation where the manufacturer can freely market the drug, replied Shri Bhushan to a question on approval for use of Itolizumab drug for COVID-19 patients. In this context, DG, ICMR stated, Tocilizumab and Itilozumab are being thought to prevent cytokine storm in severe COVID-19 patients. However, these drugs have not yet demonstrated mortality reduction by any trial. Such trials are taking place in different parts of the world to find out about the mortality reduction power of these two drugs.
Speaking on the WHO endorsed hypothesis that coronavirus might be airborne, Prof. Bhargava said, there are suggestions that there may be micro-droplets transmission through air for which again, physical distancing and use of masks is very important and has to be continued.
Presently, stratification of data collected for the sero-survey at Delhi and its analysis is being done. 22,800 blood samples were collected for this particular sero-survey till 5th July, 2020.